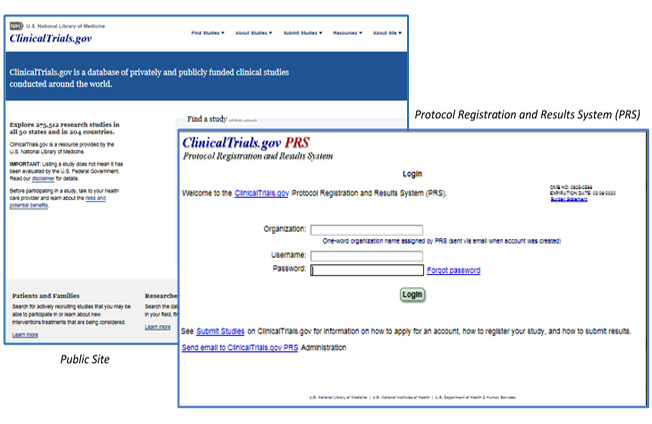
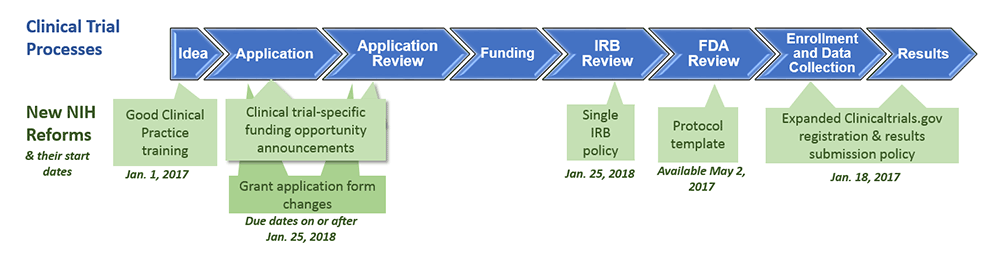
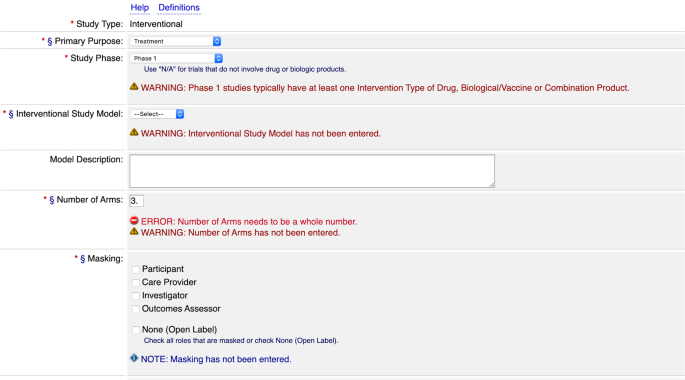
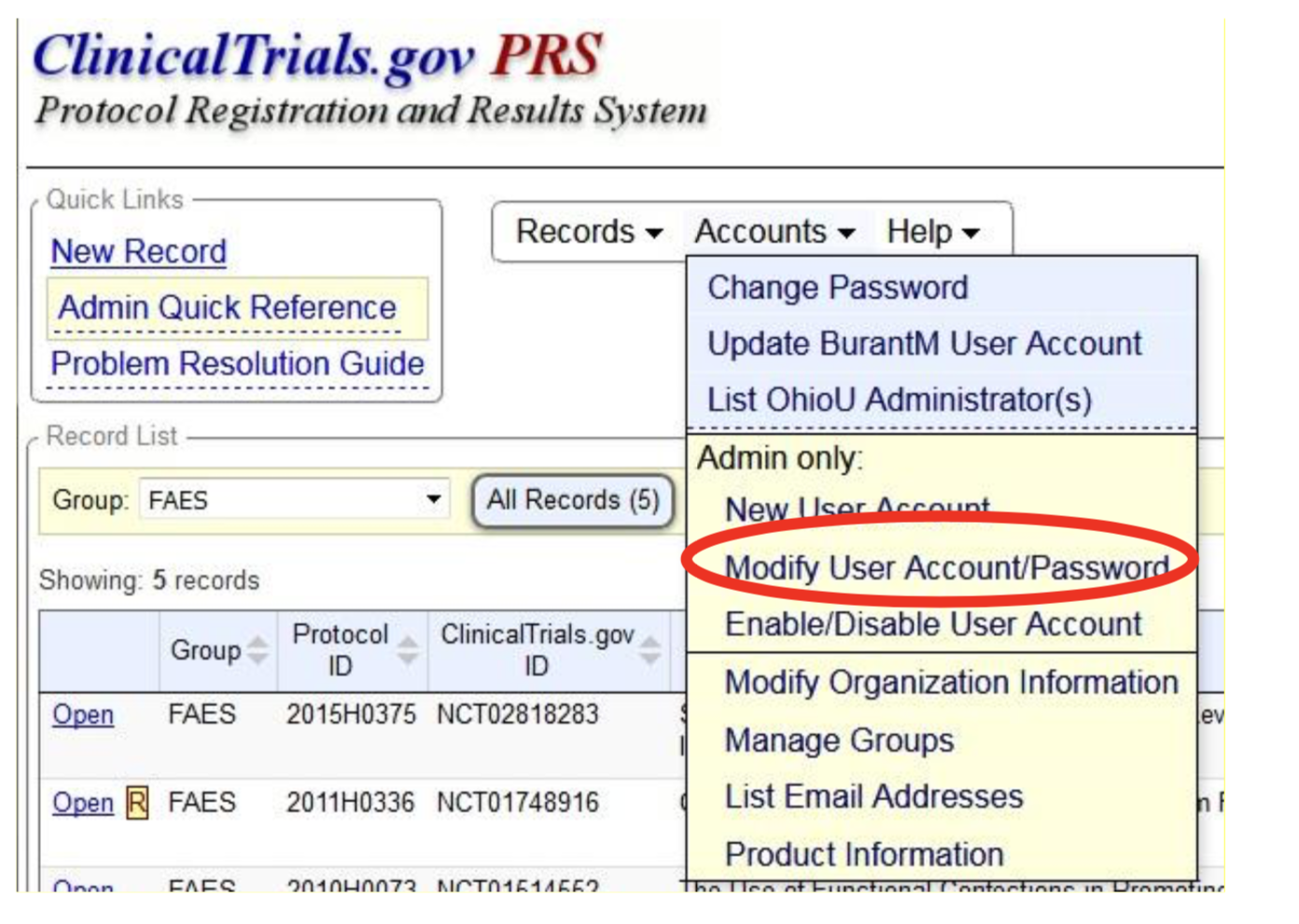
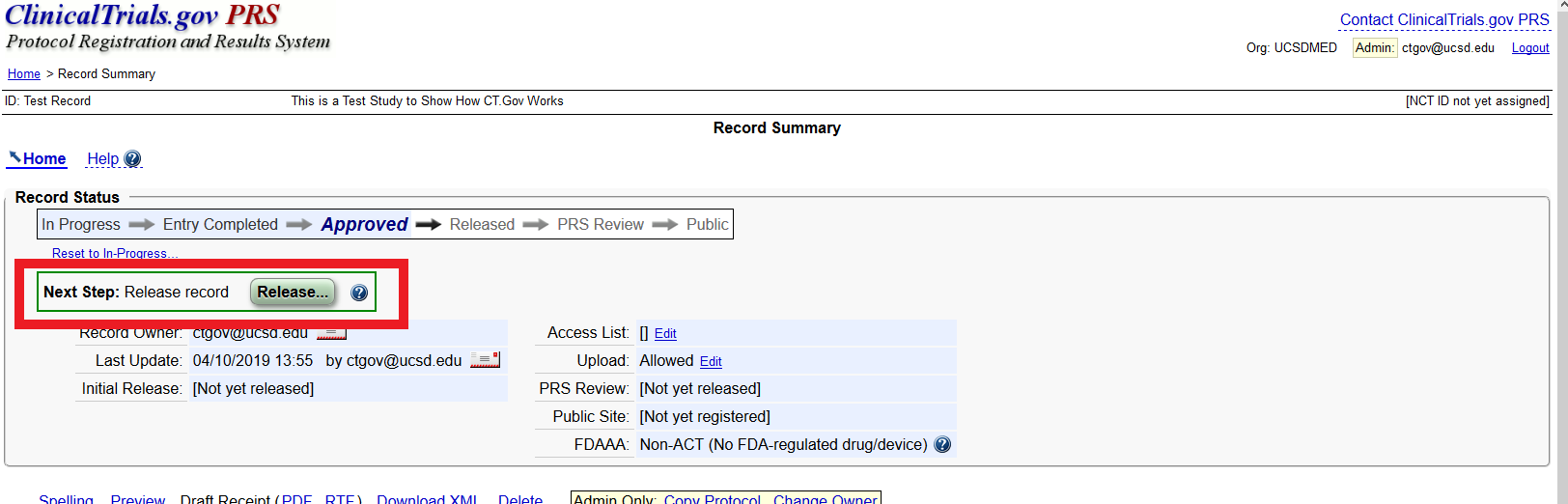
ClinicalTrials.gov trial registration submission cycle. JHUSOM: Johns... | Download Scientific Diagram

ClinicalTrials.gov: How to Register Your Trial - Clinical and Translational Science Institute - University at Buffalo
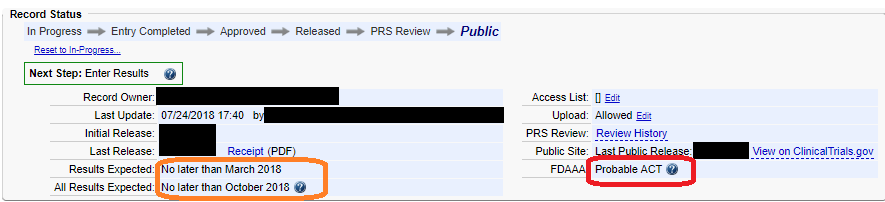
ClinicalTrials.gov Tip of the Week: Seife et al. v. HHS et al.: Results Must be Submitted for “pACTs” to Clinical Trials.gov