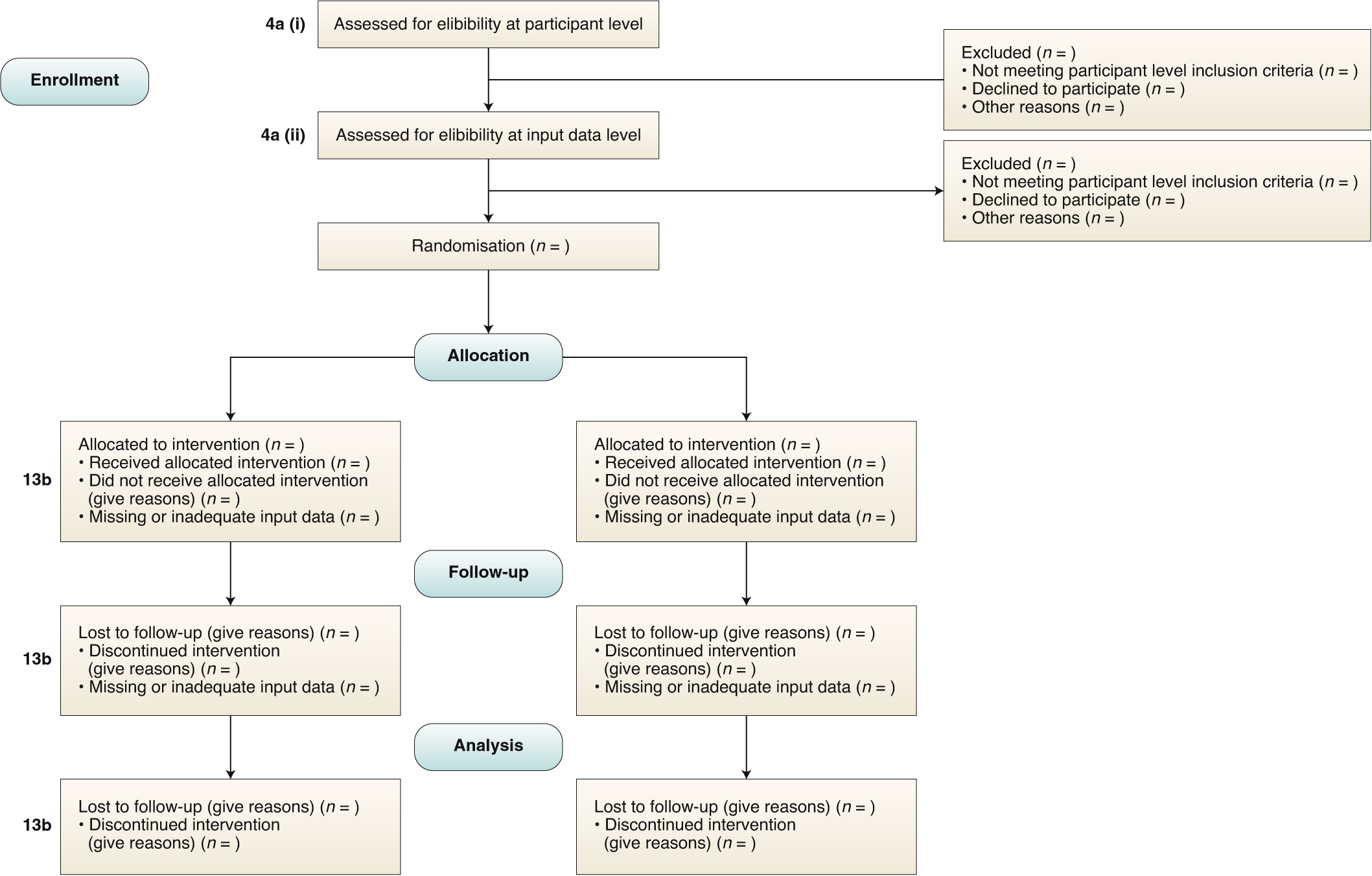
Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension | Nature Medicine

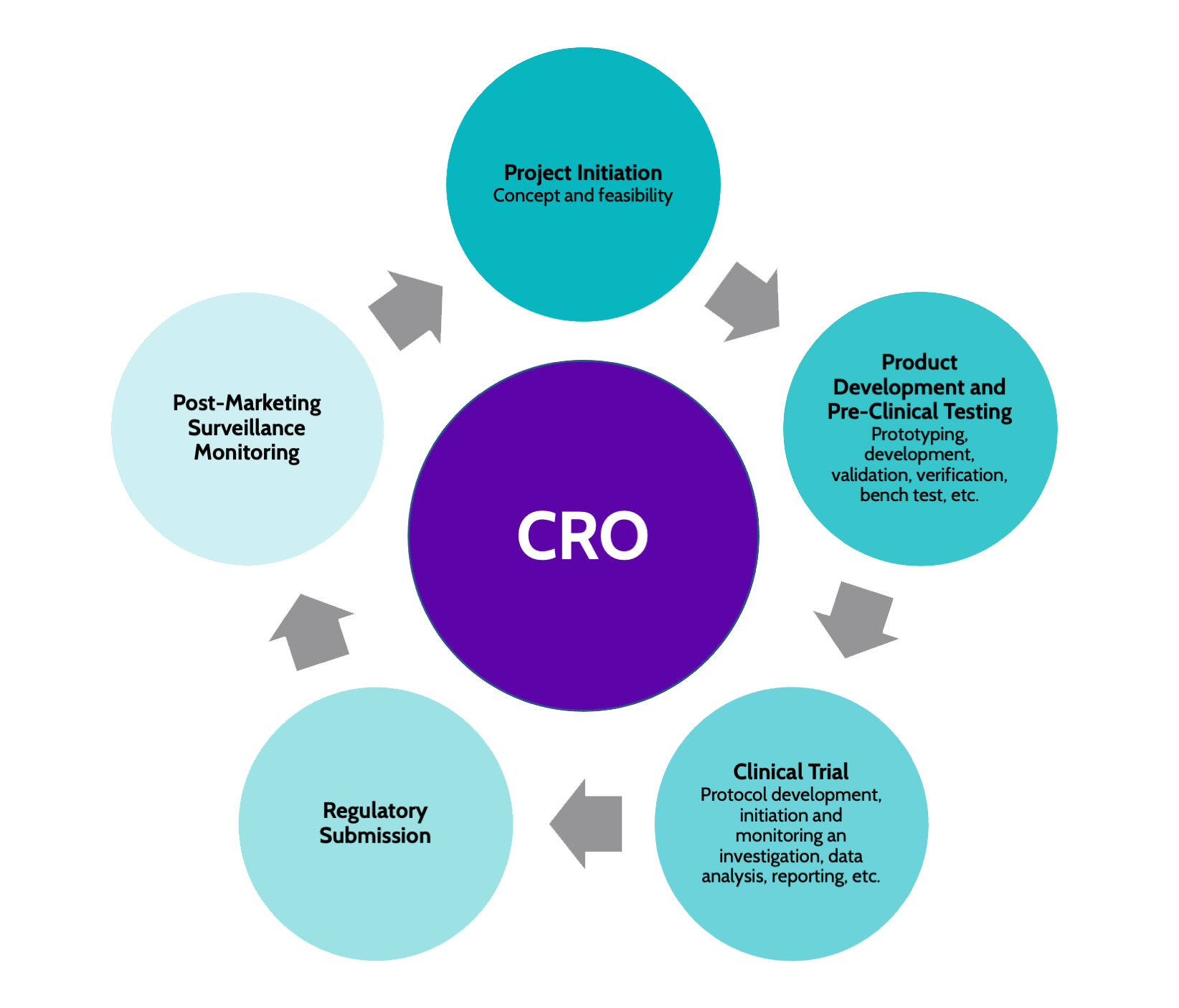
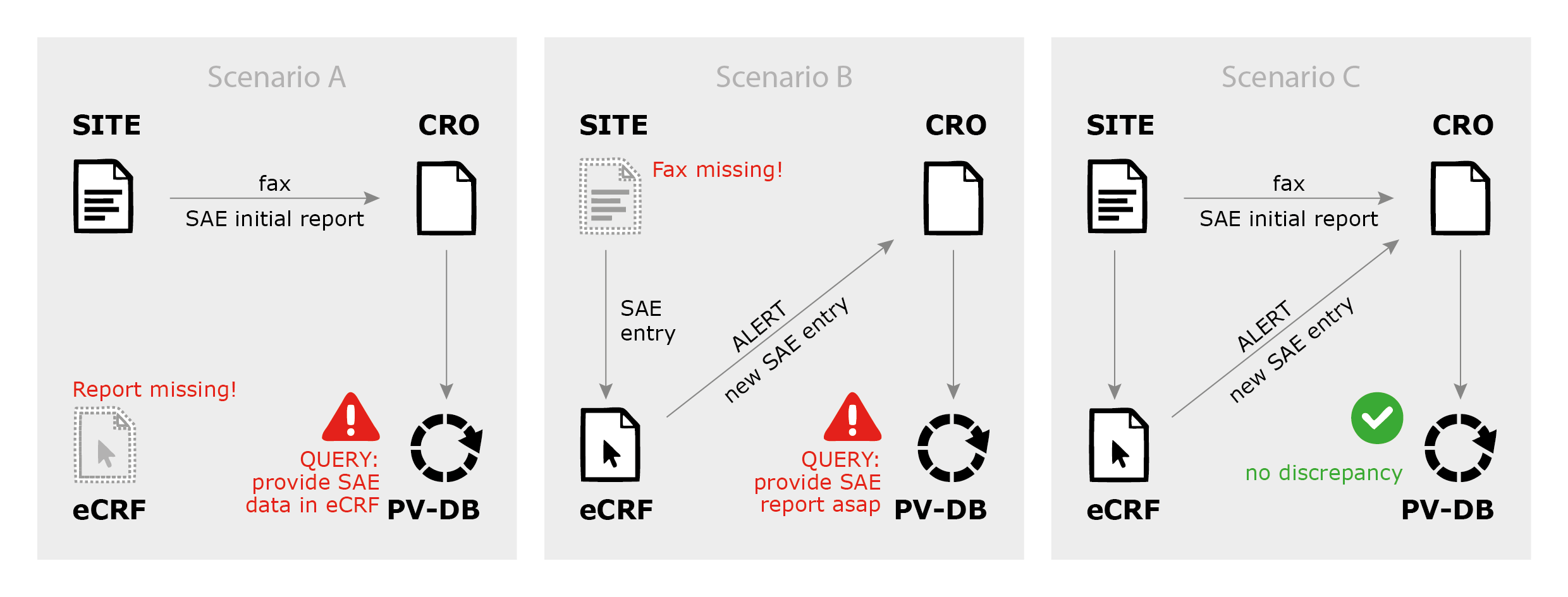
Process of reporting serious adverse events (SAE) during a regulatory... | Download Scientific Diagram

Safety Reporting Overload in Clinical Trials: FDA and Site Perspectives on Overreporting of Adverse Events | CenterWatch

Clinical Trials: A Practical Guide to Design, Analysis and Reporting: Wang, Duolao: 9781901346725: Amazon.com: Books
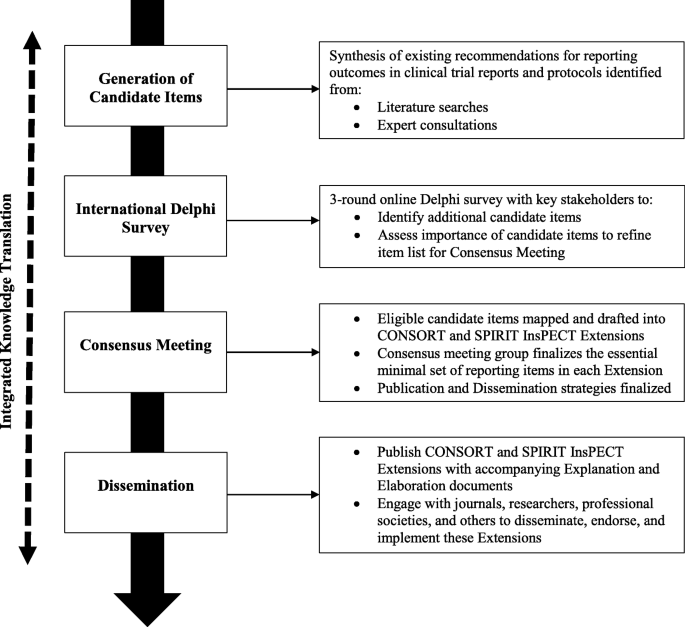
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text

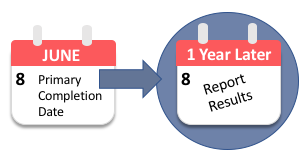
Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet

Months and Severity Score (MOSES) in a Phase III trial (PARCER): A new comprehensive method for reporting adverse events in oncology clinical trials - eClinicalMedicine